
Mårtensson, "Core-Level Binding Energies in Metals," J. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978. This bonding behavior is the result of carbon's electron configuration and electronegativity: Carbon's ground-state electron configuration of He. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5). The data are adapted from references 1-3. I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data.
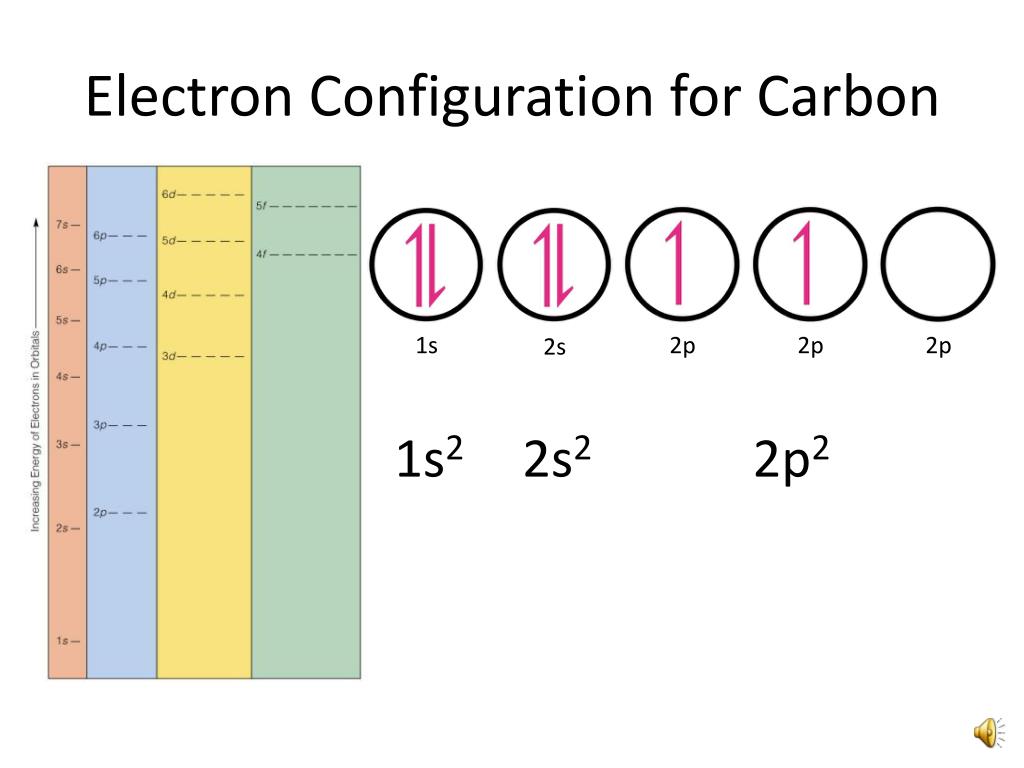
The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules relative to the Fermi level for metals and relative to the top of the valence band for semiconductors. The ground-state electronic configurations of atoms of these carbon group elements show that each has four electrons in its outermost shells. All values of electron binding energies are given in eV. an excited state of a carbon atom The ground state configuration is the lowest energy, most stable arrangement.

1967, 47, 1300.Įlectron binding energies Electron binding energies for carbon. Write down the electronic configuration of the ground state for sodium (Z11). These effective nuclear charges, Z eff, are adapted from the following references:


 0 kommentar(er)
0 kommentar(er)
